The final part of our public engagement event series took place on 26 January. This event was organised by the Progress Educational Trust (PET) and chaired by Sarah Norcross, who has been involved in several other of our public engagement activities. PET has a substantial track record of engaging in debates about IVF treatment add-ons and it was a privilege to partner with them for this event. Norcross’ review of the event in BioNews sets out some of their previous work in this area.
The event was entitled Adding Up What We Know: A Global Perspective on Fertility Treatment Add-Ons and it was opened by Kersti Lundin, the previous chair of the European Society of Human Reproduction and Embryology (ESHRE). Lundin spoke about the work that ESHRE is currently undertaking to update their good practice recommendations for IVF add-ons. ESHRE’s working group has identified 33 add-on interventions, which are categorised into four groups: diagnostic tests; laboratory interventions; clinical management; and selective add-ons. Lundin opened on a positive note by emphasising how add-ons can often be beneficial, despite often being surrounded by negative press.
Categorising add-ons is always going to be challenging, Lundin explained. Whereas add-ons are usually defined as optional, there are some interventions that are necessary for certain groups of patients. ICSI is an example of a procedure that may be considered optional or unnecessary for some but essential for others, such as couples with male-factor infertility. Lundin emphasised the role of patient-facing fertility professionals and the importance of giving patients realistic expectations as well as evidence-based advice about treatment safety.
Next, Manuela Perrotta presented some key findings from the Remaking the Human Body project. She highlighted how both IVF patients and professionals in our UK-based research had very diverse interpretations of what counts as an add-on, yet the element of financial cost to the patient was often raised as a key defining factor.
Following Lundin, Perrotta also highlighted how professionals and patients appreciate how certain add-ons may be beneficial for certain sub-groups of patients, which complicates the standardisation of guidance about add-on effectiveness. Based on research findings, Perrotta presented three recommendations for how to support patient decision-making in IVF: firstly, to coordinate and increase the visibility of already available information on add-ons; secondly, to include further information about various criteria beyond live birth rate; and thirdly, to provide further layers of information about the evidence that is available to support the use of add-ons. These recommendations reflect those made in our written evidence for the government and are further discussed in this blog post.
Moving on to a different geographical context of IVF, Sarah Lensen reflected on the place of add-ons in Australia and New Zealand. Limited data on the availability of add-ons in these countries led Lensen and colleagues’ research into how add-ons are marketed on fertility clinic websites. She found that the websites for 31 out of 40 surveyed clinics (or 78%) listed the availability of one or more add-on(s) at that particular clinic. The most commonly advertised add-ons were PGT-A, time-lapse embryo imaging and assisted hatching. Many of the add-ons advertised (77%) were accompanied by claims of benefit, such as claims of improved pregnancy chances.
A follow-on study by Lensen and colleagues surveyed IVF patients and found that the vast majority (at 82%) had used one or more add-on(s), and many of these had come at an additional financial cost to the patient. Notably, around half of participants had learned about add-ons from fertility specialists, rather than seeking this information on their own accord. Following this, the study found that many patients did not feel alone in their decision about whether or not to pursue particular add-ons, but that this was a decision that they shared with fertility specialists.
Satu Rautakallio-Hokkanen, chair of Fertility Europe, presented the results of a small survey of Fertility Europe members. In this survey she found that over half of respondents had learned about add-ons from online research, and about a third from private fertility clinics. She noted that many of the respondents were not familiar with add-ons, or perhaps they were unclear about how add-ons are defined. Rautakallio-Hokkanen reflected on why fertility patients are often willing to try unproven add-ons. She noted how patients might focus on the potential benefits, however tenuous, at the expense of considering the potential for risk or harm. Moreover, she reflected on how add-ons can make patients feel more hopeful about their treatment, especially patients who feel that they have exhausted all other options. This point spoke closely to our research findings, which highlight the importance of considering patients’ decision-making in relation to their broader experience and stage of treatment.
In the fourth presentation, entitled The Ethics of ‘Add-ons’, Sigal Klipstein discussed the challenges of assessing the science behind add-ons. Innovation has to start somewhere, usually with a theory or anecdotal evidence, which is then pursued through experimentation. This is a necessary step before new treatments can become part of standard care, but it raises important ethical questions about patients’ involvement in the early development of new interventions.
Klipstein emphasised the difficulty of engaging in conversations with patients about the risks vs. potential benefits of certain add-ons, or in conveying exactly what is meant by an unproven or experimental treatment. By undertaking certain treatments, the patient may unknowingly be the research subject. Others are, of course, fully aware that they are participating in an experimental treatment and are willing to take a high risk, even when there is potential for harm. Klipstein closed her talk by posing a series of open questions, including whether or not physicians are obligated to present ‘options’ to patients, especially when it is their professional assessment that the treatment will not offer any benefit to the patient or may even harm their chances of pregnancy.
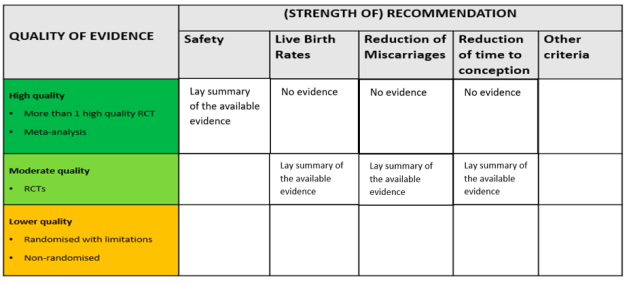
The final presentation of the evening was by Peter Thompson, chief executive of the Human Fertilisation and Embryology Authority (HFEA). Thompson spoke about the work that the HFEA has done on add-ons, which started in 2016, and their ambitions for the future. On the agenda is a review of the HFEA guidance for patients about add-ons, especially the traffic light system. He explained the challenges of providing useful information for patients in a field that is characterised by rapid change. Speaking to the previous presentations, Thompson discussed the evidence base that the HFEA uses to evaluate add-ons. Currently, add-ons are assessed based on whether there are randomised control trials that show they improve live birth rates. Yet Thompson noted how there are other measures that are relevant to certain patient groups, such as whether an add-on reduces miscarriage rates, and there are also other kinds of evidence that can be valuable. Big data, for instance, is a resource that has the potential to provide robust indications of effectiveness but is not currently being utilised in this way. The HFEA’s work on this continues and will be going out to consultation in 2022.
We would like to thank PET for organising such a stimulating event and all the speakers for their contributions. A video of the panel discussion will be made available by PET in early 2022.