Over the past few months, the research team has participated in a number of events where we have discussed our findings and engaged in stimulating conversations with IVF patients and professionals (you can see a list of our past events and links to event reports on the Events page). In many of these conversations, we offered reflections based on the results of our social science research project, often focused on the debate on evidence and treatment add-ons. As this debate is very polarised, we have sought to highlight how our findings show a variety of perspectives on add-ons and their use, from the points of view of both professionals and patients. For instance, in our written evidence to the UK government’s Women’s Health Strategy, we underlined how the patients we interviewed expressed concerns regarding the abundance of information available online on IVF treatments (and especially add-ons) from multiple sources. Patients are exposed to a variety of claims without being able to assess their reliability. In contrast, the HFEA website offers impartial, accurate and up-to-date information, but this is currently limited to the evidence available to determine whether, for most fertility patients, a certain add-on is effective at improving the chances of having a baby.

This tool offers great clarity in terms of available evidence for both professionals and patients, but, as the evidence supporting add-ons is still scarce or of low quality, none of the add-ons on the list are currently green. In addition, and in some circumstances, add-ons may be offered for reasons other than to improve the chances of having a baby. To enable patients to better-understand the risks and potential benefits for each add-on, in April 2021 the HFEA included a list of questions that patients might ask their clinicians when discussing add-ons. These questions include a variety of aspects, such as: Is there good-quality evidence to back this up? Are there any associated risks or side effects from having this add-on? Am I assured that it is safe? and Do I know about any possible risks?
Both in our discussions with patients during our The IVF Experience events (which you can read about here and here) and in our interview with BBC reporter Sophie Sulehria as part of the Fertility Show’s live seminar series, we discussed these same issues, and how our research findings can support patients’ informed decisions. In particular, in our interviews with IVF professionals, different understandings of the meaning of the HFEA traffic light system emerged. Some interviewees understood the add-ons marked red as the problematic one, while they were more lenient toward those marked amber as they considered these promising. Other professionals, instead, were more worried about the add-ons marked amber. For this group in fact, add-ons marked red are clearly-identifiable as lacking supporting evidence, while amber ones might give patients the illusion of being more effective, while in actuality there is, equally, no evidence supporting them. Similarly, our interviews with patients show very different understandings of what constitutes “evidence”. Josie Hamper and I are currently working on an academic journal article that discusses four different approaches to evidence in relation to IVF treatment add-ons. While the majority of our patient participants preferred to delegate evaluations of evidence in IVF and follow the advice of their clinic or consultant in relation to treatment decision-making, other interviewees had a more direct assessment of evidence: some engaged in critical evaluations of evidence; others acknowledged the complex process of making evidence; and others relied on embodied experiences of evidence. With such a variety of points of views, a discussion on what kind of misunderstandings these may create with health professionals and institutional bodies is needed. We attempted to foster this discussion with one of our video animations: What is Evidence in IVF?
Drawing on our research and these discussions, we elaborated a proposal that goes in the direction of offering more information to patients, that we presented in our last workshop in collaboration with PET. In line with the ongoing HFEA discussion on how best to evolve the rating system for add-ons information and to consider the strength of the evidence base for each add-on, we suggested two main additions.
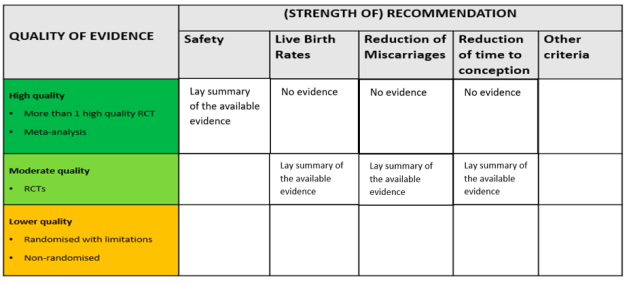
Firstly, we suggest including other criteria of evaluation for each add-on, including not only the available evidence on the ability of an add-on to improve the chances of having a baby, but also other relevant aspects for patients. This might include their safety and other potential effects on patients’ experience of IVF; for instance, the reduction of miscarriages and the reduction of time to conception. This would offer concrete support to make informed decisions for all the patients who are considering including add-ons in their treatment for reasons other than, exclusively, so as to improve the chances of having a baby. In addition, this would offer all patients more detailed information on each add-on, which they can then further discuss with their consultant.
Secondly, we suggest including additional indications on the quality of evidence available. Considering the current lack of quality evidence (i.e., more than one high quality RCT), including information regarding lower quality evidence – while highlighting that this evidence is not the best possibly available – would benefit those patients who need more clarity on why some recommendations are offered. In this case too, having reliable and detailed information available would enable patients to engage in conversations with their clinics on what the good-quality evidence they use to back up the use of add-ons is.
We anticipated two main disadvantages on offering such a detailed level of information: first, collecting and evaluating these different forms of evidence is time- and labour-intensive, and it would be a “village” effort requiring the collaboration of many members of the scientific community; second, making publicly available a summary of the currently available evidence would require agreement on a number of principles on what evidence should be included and what is too low-quality to be considered. Based on our findings, we believe being able to receive information from one reliable source would benefit many patients who are currently having to navigate information from a vast range of sources, many of which are less reliable.
During the workshop, an interesting discussion emerged on the use of the term “safety” among the criteria we suggested. For some workshop participants, this term is too ambiguous, means different things for various people and would be difficult to implement in terms of assessment. A suggestion by Katy Linderman, one of the guest speakers at the event, was to offer additional information on the potential “harm” that add-ons might have, including the risk of reducing the chances of having a baby through IVF. In line with the current HFEA discussion, it is extremely important to distinguish between the lack of evidence on a certain add-on, and the available evidence that an add-on is not safe (i.e. might somehow harm patients or reduce rather than increase their chances of having a baby through IVF).
Based on our research results and expertise, it is important for us to clarify: our proposal to share publicly the available evidence to best support informed decisions focuses on the content of the information that should be shared, rather than on how this is communicated. For this reason, we are not suggesting changes in colours or to add symbols in the visual representation of a ranking systems. Other research groups working on the best ways visually to share information are better positioned than us to provide recommendation on this.




